News and Updates
Letter from the Director - 2020 Q3
September 16th was my 2-year anniversary of coming to the NIH. When I reflect on what we have accomplished together during these past 2 years, it is no wonder that sometimes I feel tired….as I am sure you do also. The road has been and still is bumpy, although I think the potholes are less deep and not as frequent. There is no doubt the pandemic has adversely impacted the ability of our office to consolidate and streamline its operations and develop the critical team structures that are needed to function optimally. Despite this, we continue to make progress and are in every way I can think of a totally respectable IRB, staffed by dedicated, committed professionals.
Recently, the leadership of OHSRP came together and developed a vision statement.
We will promote the safe and ethical conduct of human subjects research by
- providing timely, consistent and compliant reviews
- educating our community
- communicating effectively and responsively
- collaborating with stakeholders
and thus, will be recognized as national leaders in human subjects protections.
We think this captures well what we believe it means to be the best IRB. We evaluate every decision we make against this vision to assure it is in alignment.
We have begun the process of investigating whether we should replace our current electronic IRB submission system, iRIS, with another system. Having a user friendly, streamlined IRB submission system aligns well with our vision by facilitating timely, consistent and compliant reviews. Believe me, we understand the impact of even thinking about such a change, and it is not something we are taking lightly. There are several reasons that we are considering this change. First, user satisfaction across all user groups for iRIS is very low. When we surveyed the NIH community, 70%of researchers, 62%of IRB members and 83%of IRB staff disagreed or strongly disagreed with the statement “Overall I am very satisfied with the iRIS system”. Second, the workflow processes within the system are such that they significantly hinder our review process. In addition, the system is designed in a way that makes it very difficult to accurately document important regulatory determinations. For these and several other reasons, we feel it is important to at least explore if there is a system that can better serve all of our needs. Rest assured, we will engage and collaborate with all stakeholders throughout this process. We will be presenting a session via videocast on October 6th to discuss this project in greater detail. Please be sure to tune into this to learn more.
The NIH Intramural Research Program is now a signatory to the SMART IRB Master Reliance Agreement. We are now part of a collaborative of 800 institutions nationwide that have signed the agreement. What this means for our researchers is that when we are participating in multi-site research that uses a single IRB, if our partners are also SMART signatories, we do not need to negotiate a separate reliance agreement. The SMART agreement serves as the reliance agreement. While this does not remove all the barriers to single IRB review, our hope is that it will significantly reduce burden and streamline the process. Our heartfelt thanks to all who worked so hard to get the agreement to a place where NIH could sign.
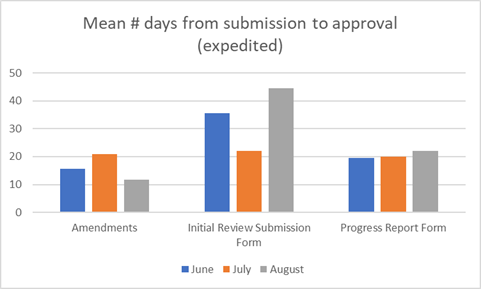
Our key metrics for the last period are shown below.
Letter from the Director - 2020 Q2
We are approaching the 6-month mark for the consolidation of all the legacy IRBs into the new NIH Intramural IRB (NIH IRB). I know that this has not been the easiest transition for the NIH community, and I thank you for your support and patience as we continue to build our new organization. Our goal is to serve the intramural research community by providing optimal protections for our research subjects and facilitating the ethical conduct of the life-saving research conducted here at NIH.
Despite the challenges of an entirely remote workforce, the IRB continues to improve its metrics. The IRB is receiving approximately 400 forms submitted each month. The mean time to approval in May for new studies, amendments and continuing reviews reviewed by the full board were 40, 46 and 28 days respectively. For forms approved by the expedited path, initial reviews were approved in 13 days, amendments in 14 days and continuing reviews in 11 days. We continue to examine our processes to identify areas for increased efficiencies.
We have prioritized all COVID research, and the mean approval times for COVID related research were 4.5 days for initial reviews, 5.7 days for amendments, 2 days for NHSR determinations and 5.5 days for institutional approvals for multi-site research in which we are relying on an external IRB. These truly amazing stats reflect the incredible hard work and dedication of the entire OHSRP staff that have dropped everything and worked many long hours to move these studies from submission to approval. I can’t thank them enough for their dedication.
We have begun exploring the feasibility of replacing iRIS with a new electronic IRB system. A steering committee made up of representatives from the ICs, the Assembly of Scientists and OHSRP have met once to discuss the process for evaluating the current landscape of systems. While we cannot have a committee that has representation from every IC, rest assured that we will be soliciting direct input from the entire research community throughout the entire process. Our goal is to find a system that serves our entire research community well, is forward looking and can grow as clinical research grows at the NIH. I expect that this will be a long process, and in the meantime, we will continue to optimize iRIS.
As I said when I first arrived here, we can only succeed as a human research protection program (HRPP) if we work collaboratively with our investigator community. I am grateful that the investigator community has been so willing to work with us and help us understand how best to serve their needs and those of our research participants. The NIH can be a challenging place from an organizational standpoint. I was asked recently, what do I know now that I wish I knew earlier. My response was that when I arrived here, I thought the NIH was like a University with 27 different departments. I now know it is more like 27 different Universities.
Letter from the Director - 2019 Q4
Dear NIH Community,
The end of the year is frequently a time for pause and reflection, so I wanted to share with you some of what we have accomplished in the past year and where we are headed. 2019 was a busy year for OHSRP with lot of change across the clinical research landscape of the NIH Intramural Research Program. Some of the accomplishments of the past year are highlighted below.
Staff
- 25 new FTE and 5 contractors hired and onboarded
Space
- Moved to new space at 6700B Rockledge and consolidating our on-campus footprint into renovated space in building 31 that was previously occupied by the CNS IRB.
IRBs
- Rolled in all legacy IRBs into the new NIH Intramural IRB
- Implemented the revised Common Rule
- Implemented the flexible IRB model with the new NIH IM IRB meeting 3 times per week in 2019
- Standardizing IRB and regulatory determinations across the IRP
- Processes and Policies
- Undertaking a complete rewrite of NIH HRPP policies. On track to complete by early 2020
- Developed new protocol and consent templates
- Revised short form consents
- Removed the “witness to the signature” requirement
- Updated exempt determination process, and put into IRIS
- No more mandatory NHSR determinations (NHSR forms now in iRIS)
Electronic IRB system
- Completed migration onto single platform
- Consolidated IT team under IRBO leadership
- Redesigning the forms, workflow and processes for enhanced efficiency
- Federated login to support external users
- Reliance/sIRB processes
- Master agreement to WIRB and Advarra put into place
- Manage exceptions to sIRB policy
- Working with all parties to sign on to SMART IRB
Compliance
- Completely new office of Compliance and Training formed within OHSRP
- Revised and implemented new policies on the reporting and review of non-compliance, non-compliance and other reportable events.
- Established the Research Compliance Review Committee
Education
- New monthly NIH wide OHSRP education series
- Implemented regular education and training for IRB operations staff, IRB members and Chairs training
What’s in store for 2020?
The upcoming year will focus heavily on process improvement. Now that the new IRB and IRB office are overseeing all research, we can direct our efforts to streamlining the review process and assuring that all protocols have the required regulatory determinations made and documented. Our goal is to minimize unnecessary burden on investigators and the IRB to allow for our energy to be focused on what matters, performing high quality reviews to make sure the best research moves forward ethically, efficiently and in a way that is compliant with all the regulatory requirements.
We will be concentrating our efforts in four major areas:
- Streamlining our internal IRB processes as well as our interaction with other clinical center offices to allow for the most efficient review process.
- Optimizing our electronic IRB submission system, iRIS, to facilitate an efficient submission and review.
- Assuring that all protocols being conducted under NIH IM IRB oversight have the required regulatory determinations made and documented in the electronic system.
- Providing educational opportunities on human subjects protections that are responsive to the needs of the NIH IRP community
I am enormously proud of all the work that the staff of the OHSRP has done to get to where we are today. There have been bumps in the road and I have no doubt new challenges will arise over the course of the next year, but also am confident that we can meet and overcome those challenges.
I am also extremely grateful for the support the leadership of the NIH has provided, and the support and patience of the entire NIH intramural research community. We could not do this without you. In the end, together we will have built the best HRPP in the country. Our patients, participants and researchers deserve nothing less.
Jonathan